With the continuous depletion of fossil energy sources like coal, oil, and natural gas, coupled with the escalating environmental and climate issues stemming from their combustion, China’s green and sustainable “dual-carbon” development strategy underscores the importance of efficiently utilizing energy and seeking new alternative sources. Biomass energy, being renewable, low-pollution, and abundantly available, has garnered significant attention globally. Biomass encompasses all organisms generated through photosynthesis, including plants, animals, and microorganisms. This form of energy stores solar energy in biomass as chemical energy and is derived from the photosynthesis of green plants. Biomass energy can be converted into conventional energy fuels, making it a promising renewable energy source. In general, biomass energy involves utilizing natural vegetation, animal waste, and organic urban and rural residues for energy production [1, 2].
Biomass energy offers a clean, safe, and readily available option [3-6], aligning well with China’s “dual-carbon” development strategy. Its development and utilization can address China’s energy challenges and promote energy source diversification in response to tight energy supplies. The prevalent biomass energy conversion technology is direct combustion, boasting good fuel adaptability, requiring simple raw material processing before incineration. Currently, the most established and widely adopted biomass utilization technology is biomass direct combustion power generation or steam-based systems [7]. However, the progress of this technology has been impeded by the issue of high-temperature corrosion on the boiler heating surface. This corrosion primarily occurs through two main processes:
- Biomass contains a significant amount of alkali metals. During combustion, alkali metals form chlorides that condense on the heating surface tube walls, reacting with the surface oxide film and initiating high-temperature corrosion.
- Gaseous HCl and Cl2 in the flue gas exhibit strong penetration capabilities, disrupting the protective film on metal surfaces and causing direct internal metal reactions, which accelerate corrosion rates with rising temperatures [8].
As a result, biomass boiler heating surfaces suffer severe high-temperature corrosion during operation, leading to thinning of the heating surface tubes [9] and even pipe bursting accidents, thereby affecting the boiler unit’s normal and stable operation. Since most biomass boilers use straw biomass as fuel, the high content of alkali metals and chlorine elements in straw exacerbates boiler tube thinning during operation. To address this issue, this paper analyzes the causes of corrosion on the boiler tube heating surface under biomass incineration conditions by examining the ash, slag, and corrosion products. The findings lay the groundwork for subsequent boiler tube protection strategies.
Test Materials and Test Methods
1.1 Test Materials
During the shutdown of the biomass boiler, a portion of ash and slag is collected from the furnace for composition analysis. When the heating surface requires an overhaul due to severe corrosion that does not meet usage requirements, specific heating surface tubes are intercepted and sampled through wire cutting to obtain samples with dimensions adhering to the test size of 10 mm × 10 mm.
1.2 Testing Method
The intercepted samples from the heating surface tubes are carefully set, ground, and polished. Subsequently, a Tscan VEGA TS scanning electron microscope, along with the accompanying energy spectrometer (EDS), is employed to examine the cross-sectional morphology and composition of the specimens. Additionally, the ash and slag collected are subjected to composition analysis.
Boiler Tube Heating Surface Corrosion Analysis
2.1 Analysis of Furnace Ash and Slag
The furnace ash appears mostly in the form of powder, with a small fraction present as rods. Energy spectrum analysis of the entire ash, as depicted in Figure 1, reveals the elemental content displayed in Table 1. The primary elements identified are C, Si, and O, with a Cl content of 0.36 wt.%. The S element is below the detection limit, and a small amount of alkali metal elements like K and Na, as well as other metal elements like Ca, Fe, and Al, are also present. Within the rods, O and Si contents show a significant decrease, while Cl content increases to 1.48 wt.%, and S content is detected at 0.38 wt.%.
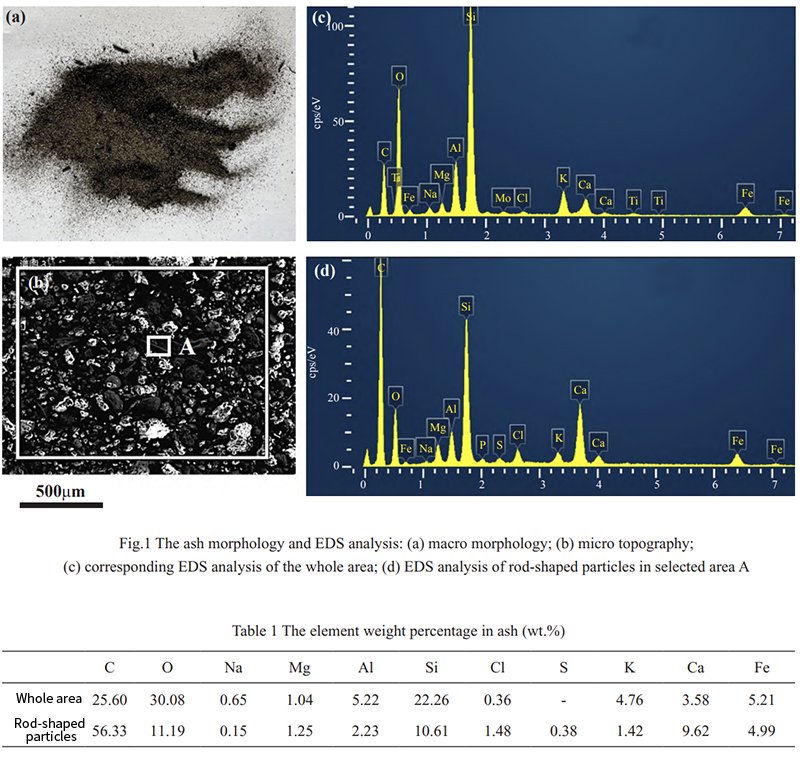
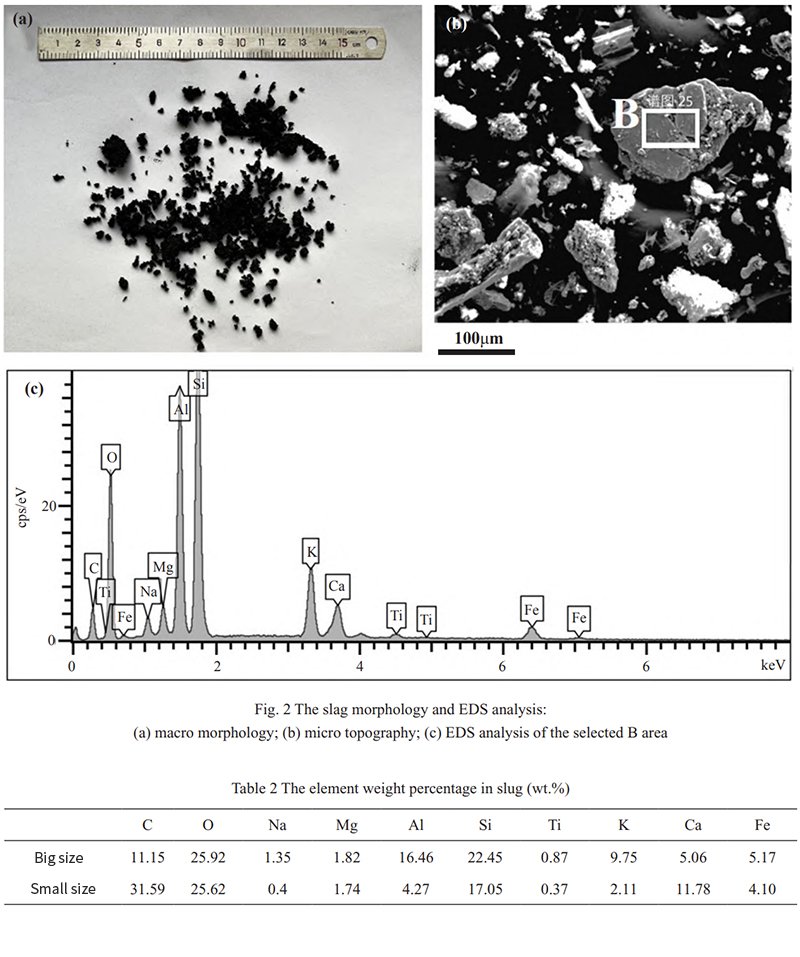
The slag, observed to be black-gray and granular, consists mostly of particles with diameters ranging from several millimeters to some exceeding 10 mm, as shown in Figure 2(a). Scanning electron microscopy and energy spectrum analysis results are displayed in Figure 2(b) and Figure 2(c), respectively. Compositional detection and analysis of the slag with varying sizes are presented in Table 2. The primary components of the slag include alkali metals like Al, Ca, Fe, and other elements such as Si and C, existing in the form of oxides. Notably, the larger-sized slag particles exhibit higher C content, which stems from insufficient slag combustion. Importantly, no detectable Cl elements are found in the slag, indicating that during biomass combustion, Cl elements are either in the form of gaseous state, molten salts, or fly ash attached to the surface of the tube wall.
2.2 Analysis of the Corrosion Layer of the Heating Surface Pipe
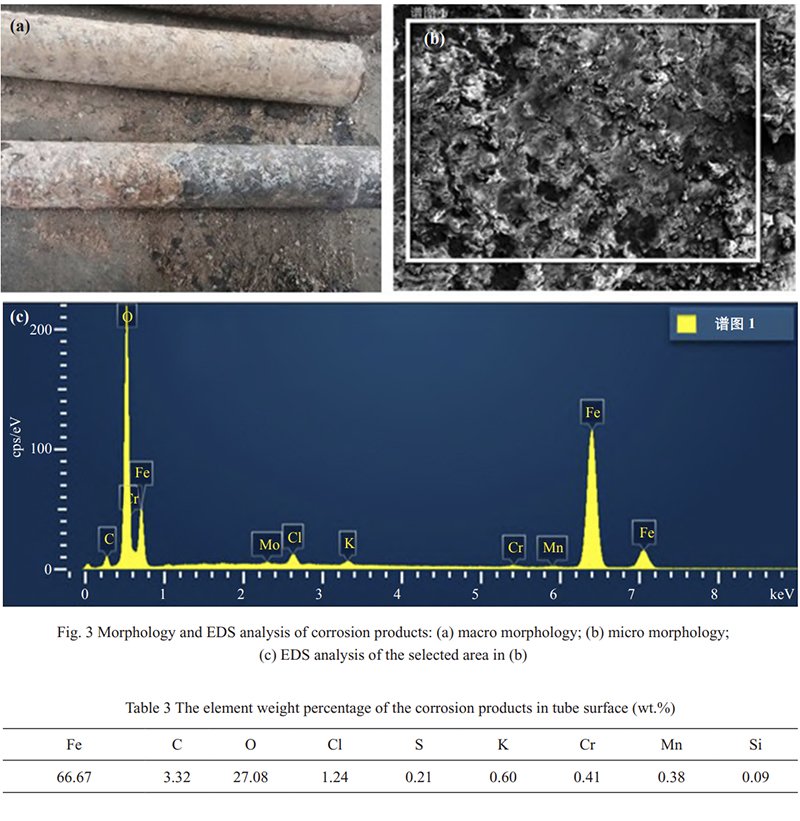
Upon examining the sampled tube’s appearance, it is evident that the outer surface of the boiler tube is covered with a substantial amount of corrosion products, featuring prominent flaky spalling layers. A small quantity of corrosion products is extracted from the heating surface tube for elemental analysis. Figure 3 illustrates the macroscopic morphology and elemental analysis of the corrosion products on the heated surface tube, while Table 3 presents the composition test results. It is observed from Figure 3 and Table 3 that the corrosion products contain a significant amount of corrosive elements, particularly chlorine and sulfur, with chlorine content reaching as high as 1.27 wt.%. Consequently, high-temperature chlorine corrosion is identified as the primary cause of boiler tube corrosion thinning during biomass boiler operation.
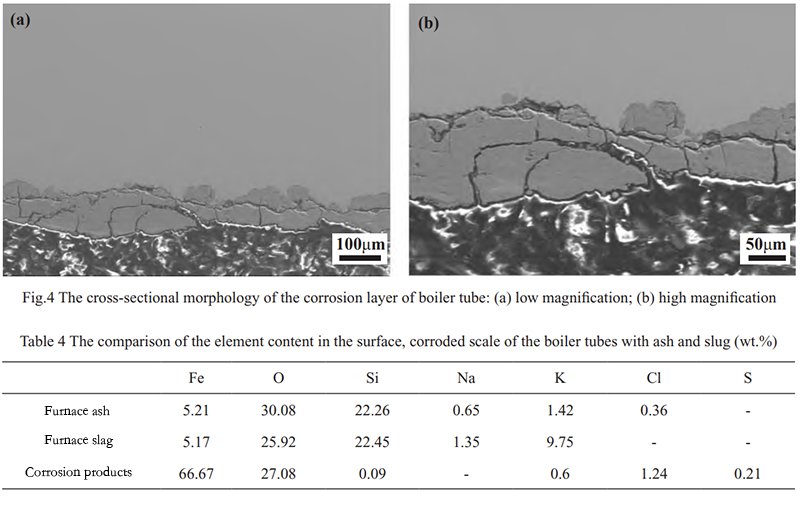
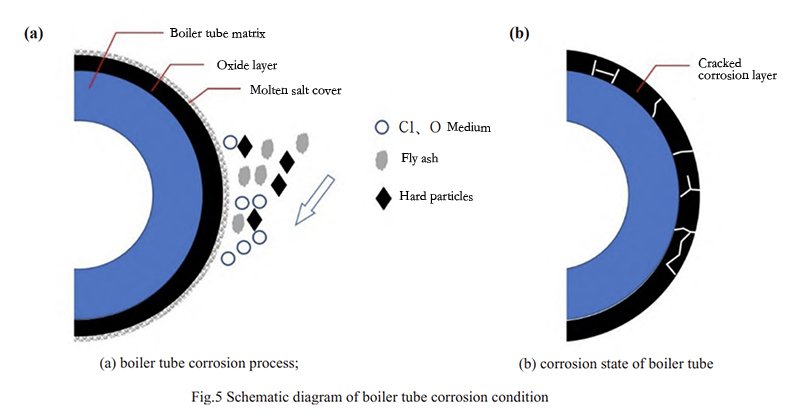
To delve further into the impact of high-temperature chloride corrosion on the heating surface tube, a cross-sectional observation is conducted, analyzing the characteristics and component distribution changes within the boiler tube’s corrosion layer. The morphology of the corrosion layer in both low and high magnifications is displayed in Figure 4. As observed from Figure 4, the corrosive cracking and peeling occur on the surface of the heated surface tube. The main reason is the exposure of the outer wall side of the tube wall to the corrosive elements present in the flue gas. Before being put into operation during the tube wall manufacturing stage, a layer of oxidized film naturally forms on the metal surface, forming a dense protective film composed of Fe3O4 and Cr2O3, providing corrosion resistance.
However, during boiler operation, the flue gas carries corrosive elements that deposit onto the oxide layer’s surface, leading to infiltration adhesion. Subsequently, gas-phase corrosion, molten salt corrosion, and localized electrochemical corrosion, among other complex and continuous physicochemical corrosion reactions, cause the detachment of the protective Fe3O4 and Cr2O3 film from the metal surface. The higher the temperature, the faster the corrosion of the heating surface [10, 11]. Consequently, clear interfaces are observed between the corrosion layer and the substrate, with numerous transverse and longitudinal cracks within the corrosion layer, accelerating thinning through peeling under the scouring effect.
2.3 Corrosion Mechanism Analysis
The composition of the boiler tube heating surface corrosion products, as well as the ash and slag, is presented in Table 4. Based on the ash and slag composition analysis, it is evident that the primary corrosive element is chlorine (Cl), with low sulfur (S) content. Additionally, alkali metals such as sodium (Na) and potassium (K) can combine with Cl to form corresponding chlorides or sulfides, leading to molten salt corrosion at elevated temperatures. Under the high-temperature operating conditions of the boiler tube, the presence of Cl elements and alkali metal salts accelerates the oxidation process, referred to as active oxidation, which compromises the protective effect of the oxide film. The detection of high Cl content in the surface corrosion material of the boiler tube serves as evidence of active oxidation.
The active oxidation process involves the interaction between Cl elements and the protective oxide film on the metal surface. As the temperature rises during boiler operation, Cl actively participates in the oxidation reactions, leading to the formation of volatile metal chlorides. This phenomenon destabilizes the protective oxide film, rendering it porous and more susceptible to further corrosion attacks. The combination of Cl elements and alkali metal salts further exacerbates the corrosive environment on the heating surface, causing a significant loss of the protective layer. Consequently, the boiler tube experiences accelerated corrosion thinning, reducing its structural integrity and potentially leading to tube bursting incidents.
In conclusion, the corrosion mechanism on the heating surface of the boiler tube is primarily attributed to high-temperature chloride corrosion facilitated by Cl elements and alkali metal salts. The active oxidation process, initiated by Cl during boiler operation, weakens the protective oxide film, ultimately resulting in severe corrosion thinning and compromising the boiler’s operational stability. Understanding these corrosion mechanisms is crucial for developing effective strategies to protect and maintain the heating surface tubes in biomass boilers.
Boiler Tube Corrosion Prevention Strategies and Case Study
Temperature stands out as the most influential factor affecting the corrosion rate of boiler tubes. The fundamental approach to addressing the corrosion issue and extending the tube’s life is to reduce the operating parameters of the boiler. However, significant parameter reduction adversely impacts the thermal efficiency of the boiler, subsequently affecting the overall efficiency of the power plant. Therefore, in boilers operating at high parameters, the best economic and social benefits can be achieved by enhancing the corrosion resistance of the alloy steel tubes through the substitution of current boiler piping steel with more corrosion-resistant alternatives. Nonetheless, replacing the entire heat exchanger tube would entail substantial investment for the power plant. Consequently, the optimal strategy involves surface protection through sacrificial coating.
This approach entails applying a highly corrosion-resistant alloy coating on the surface of the boiler tube as a sacrificial layer. The coating safeguards the boiler tube body from corrosive media during incineration, gradually corroding itself while protecting the tube. Periodic furnace shutdowns are performed to monitor coating thickness and other corrosion characteristics, allowing for the timely spraying of new coatings, ensuring cyclic protection of the boiler tube body.
Selecting suitable corrosion-resistant alloy coatings for biomass incineration environments necessitates considering heat transfer efficiency. In this regard, coatings made of metal alloys are typically preferred over ceramic coatings, which have low thermal conductivity and large thermal expansion mismatch with the boiler tube substrate. Given that biomass incineration environments predominantly lead to boiler tube thinning due to active high-temperature chloride corrosion, coupled with the high-speed scouring effect of fly ash, the ideal coating needs to exhibit resistance to high-temperature chloride corrosion and a certain degree of hardness, while considering corrosion and wear resistance.
The corrosion resistance of metal chlorides in chlorine environments is determined by their vapor pressure. To achieve resistance to high-temperature chloride corrosion, it is advantageous to select coating materials like nickel-chromium (NiCr), which exhibit lower volatility compared to iron chloride (FeCl), thereby reducing the Cl element’s participation in the corrosive cycle and slowing down corrosion. As such, NiCr can be chosen as the main component of the coating, complemented by metal or non-metal elements that facilitate the formation of a hard phase.
To tackle high-temperature corrosion of the heating surface in biomass boilers, corrosion-resistant alloy wire and anti-corrosion spraying treatments are employed. In on-site boiler anti-corrosion spraying for power plants, the process involves sandblasting and descaling, surface activation, arc spraying, and sealing treatment. The sealing treatment ensures that corrosive gases in the furnace do not corrode the substrate through the internal pores of the coating, preventing coating peeling and failure.
A case study illustrates the on-site anti-corrosion spraying process for power plant boilers. Fig. 6(a) showcases a large number of corrosion products attached to the heating surface of the boiler before spraying. However, through surface cleaning, activation, and spraying treatment, the coating on the boiler tubes’ surface remains free of cracks and peeling, as depicted in Fig. 6(b). Following spraying, sealing treatment is applied (Fig. 6(c)) to block the diffusion path of corrosive atmosphere, ensuring the corrosion-resistant performance of the coating. After one year of operation in an actual biomass incineration environment, the boiler heating surface inspection (Fig. 7) reveals an intact coating surface with molten salt and oxides attached, signifying the coating’s effectiveness in isolating corrosive media and extending the boiler tube’s service life.

Conclusion
(1) The analysis of biomass combustion boiler fly ash, slag, and corrosion products revealed the presence of significant corrosive elements, particularly elemental chlorine. The chlorine content in the ash was found to be 1.48 wt.%, while in the corrosion products, it amounted to 1.27 wt.%. However, no chlorine was detected in the slag, suggesting that chlorine in its gaseous state, molten salt, or fly ash adheres to the tube wall surface during the biomass combustion process. Consequently, the primary cause of corrosion thinning in biomass boiler tubes during operation is high-temperature chloride corrosion.
(2) The interaction between chlorine, alkali metals, and other elements at high temperatures in biomass boilers leads to a series of complex physical and chemical reactions, resulting in corrosion of the boiler heating surface tube wall. The corrosive environment loosens and cracks the surface oxide film, which is further aggravated by material scouring, leading to the detachment of the oxide film and thinning of the tube wall. The coupling of high chlorine/molten salt corrosion with scouring and wear accelerates the tube wall’s thinning.
(3) To address the high-temperature corrosion problem of the boiler tube heating surface, thermal spraying technology is employed to create a corrosion-resistant metal coating. This approach, after one year of actual operational conditions, proved successful as the corrosion-resistant coating remained intact with molten salts and oxides adhering to its surface. This indicates that the coating effectively isolates the corrosive medium from corroding the tubes on the heating surface, thereby extending the boiler tubes’ service life.
In conclusion, the study highlights the significance of combating high-temperature chloride corrosion in biomass boiler heating surfaces. The application of corrosion-resistant coatings through thermal spraying offers a viable solution to prolong the boiler tubes’ lifespan, ensuring efficient and reliable operation in biomass power plants.
[Source] Wang Hao1, Liu Chengwei2, Qin Enwei3*, Wu Shuhui2, Chen Guoxing2, Ye Lin2, Thermal Spray Technology1674-7127(2022)04-0009-8
1. Jiangsu Deli Chemical Fiber Co. LTD, Suqian 223800;
2. Suzhou Nuclear Power Research Institute, Co. Inc, Suzhou 215004;
3. Suzhou Vocational Institute of Industrial Technology, Suzhou 215104
[1] Wang Jiuchen , Dai Lin , Tian Yishui , et al. Analysis of the development status and trend of biomass energy industry in China [J]. Journal of Agricultural Engineering , 2007, 9: 276-282.
[2] Li Junfeng , Wang Zhongying . Interpretation of Renewable Energy Law of the People’s Republic of China [M]. Beijing , Chemical Industry Press , 2005.
[3] Gao Yan , Zheng Shengyu . Innovative biomass energy to help the implementation of the “dual carbon” strategy [J]. Science and Technology Innovation and Branding , 2021, 12: 52-55.
[4] Li Qiang. Environmental protection and energy saving biomass circulating fluidized bed boiler [J]. Metallurgical Management , 2020, 19: 144-145.
[5] Zhou Yi , Zhang Shouyu , Langsen , et al. Research progress of biomass power generation technology in pulverized coal furnace [J]. Clean Coal Technology , 2022, 28: 26-34.
[6] Wang Haibo , Liu Haiyong . Introduction to boiler furnace type and grate of biomass direct-fired power station [J]. Science and Technology Consulting , 2019, 34: 53-55. [7] Demirbas M F, Balat M, Balat H. Potential contribution of
biomass to the sustainable energy development[J]. Energy
Conversion and Management, 2009, 50: 1746-1760.
[8] Sun Hongwei , Lv Wei , Li Ruiyang . Study on alkali metals in biomass combustion process [J]. Energy Conservation Technology , 2009, 27: 24-26. [9] Lu J, Wang Z S, Li Z S, et al. Dew point temperature ofgases with sulphur and its corrosion analysis[J]. Energy for Metallurgical Industry, 1991, 10: 54-55.
[10] Zahs A, Spiegel M, Grabke H J. Chloridation and oxidation of iron, chromium, nickel and their alloys in chloridizing and oxidizing atmospheres at 400-700 ℃ [J]. Corrosion Science, 2000, 42(6): 1093-1122.
[11] Yang C Q, Chen J Y. Waste heat utilization from municipal refuse incineration plant-high-temperaturizing and high-pressurizing of a waste heat boiler[J]. Boiler Manufacturing, 1995, 2: 50-63
DHB Boiler
Discover The Superior Quality And Cutting-Edge Technology Of DHB Boilers. Explore Our Range Of Biomass Boilers, Waste Heat Boilers, And More. Take Your Industrial Operations To New Heights With DHB Boiler.








