Biomass energy is an amazing source of renewable and clean energy with abundant resources. I mean, it’s like the superhero of green, environmentally friendly, and sustainable energy in China. The whole scientific and technological research and industrialization development of renewable energy, like biomass energy, are right up there on our national priority list. We’re talking high-tech stuff, folks!
But hold on a second, there’s a catch. Biomass has this somewhat tricky issue – it’s got a bit too much of those Cl and alkali metal elements. Now, what’s the fuss, you ask? Well, it turns out that these elements can throw a wrench in the works, particularly in the superheater area of biomass boilers. It messes with the safe and stable operation of the boiler unit. We can’t have that, right?
See, the steam temperature plays a huge role in the efficiency of power generation in a station. When the steam temperature is below 300 ℃, the power generation efficiency is only about 12%. But, if we crank that temperature up to 400 ℃, the efficiency skyrockets to a cool 21%. That’s some serious improvement, and it means more power and more money. But, and there’s always a ‘but,’ raising the steam temperature also cranks up the corrosion in the superheater piping. Superheater metal pipes tend to run about 5-20 ℃ hotter than the internal steam temperature, and that’s where the corrosion creeps in. It’s a bit of a balancing act, folks.
Now, here’s a little science for you. High-temperature corrosion is like a whole series of reactions that go down on the heated surface of metal when it’s dealing with scorching hot flue gas. There are a few types of corrosion, but we’re interested in one in particular – chloride-type corrosion. See, it’s the main troublemaker in the superheater region of biomass boilers.
So, the story goes like this: at temperatures between 300 to 480 ℃, we’re in the “not too bad” zone; a bit of corrosion, but nothing crazy. But when we hit 550 to 700 ℃, that’s where the party’s at, and by ‘party,’ I mean strong corrosion. As the temperature goes up, the superheater corrosion just gets more and more serious. It’s a bit like battling the elements to keep our power plants running smoothly, all while being mindful of efficiency. That’s the challenge we’re facing, and it’s one we need to tackle head-on.
Okay, so here’s the deal – when it comes to high-temperature corrosion caused by chlorine (Cl) at those scorching hot temperatures, there are three main ways it goes down. Let me break it down for you:
- Gas-phase corrosion: Think of this as the “atmosphere battle.” You’ve got reducing atmosphere corrosion, oxidizing atmosphere corrosion, and gaseous alkali metal chloride corrosion. What happens here is the high chlorine content in the raw materials gets all reactive in the gas phase. It reacts with the metal surfaces that are heated, causing corrosion or making it speed up. It’s like a chemical showdown in the air.
- Solid-phase corrosion: Imagine solid particles settling in like uninvited guests. This is where chloride and other stuff from the flue gas deposits on the metal surfaces. There’s also alkali metal chloride joining the party, causing corrosion by turning into metal carbide. It’s like these harmful elements condense, deposit, and speed up the corrosion process. Not a fun time for the metal alloys.
- Liquid-phase corrosion: Think of this like a sneaky liquid attack. Liquid-phase chloride corrosion occurs when harmful elements in the ash accumulate on the heated surface and form a local liquid phase. The reason for this is that metal chloride and inorganic salts in the flue gas cozy up to the pipe wall’s surface. They form a low melting point co-crystalline, which makes the ash melt at lower temperatures. This leads to a corrosive liquid phase on the metal surface, triggering electrochemical corrosion. The base of the metal alloy acts like an anode dissolving away, and it’s not good news. It combines with oxidants like O2 and Cl2 in the flue gas, oxidizing further and forming deposits or chlorides. All of this eventually corrodes the heated surfaces.
Now, these Cl-related gas, solid, and liquid-phase corrosion processes, they all play off each other, kind of like a complicated dance. And here’s the kicker – even a little S (sulfur) can jump into the mix and speed up the corrosion process. So, it’s a bit of a chemistry showdown on the superheater’s heated surface, which, over time, leads to corrosion layers building up and the pipe wall getting thinner. And when that wall gets too thin, well, you’re looking at potential disasters like pipe bursts under pressure.
So, we roll up our sleeves and dive into the study of high-temperature corrosion on the superheater pipe wall. We’re checking out how the pipe wall’s structure and performance change at different temperatures after corrosion. This is like our secret weapon to tackle high-temperature corrosion on the superheater, and it’s got some pretty crucial insights.
For this mission, we picked a 130-ton/hour high-temperature and high-pressure biomass circulating fluidized bed boiler from Jiaxing Xinjia Aisi Cogeneration Co., Ltd. as our test subject. We’re looking at the superheater made of TP347H (yeah, that’s the type of metal) with dimensions of φ38.0 mm × 6.0 mm. And we’re checking it out after 28,650 hours of heavy-duty operation. The steam temperatures we’re focusing on are 475-480 °C for the medium-temperature superheater and a sizzling 530-540 °C for the high-temperature superheater. It’s all part of our grand experiment to uncover the secrets of superheater corrosion.
Testing
So, let’s dive into the nitty-gritty of our testing:
1.1 Test Material
We’re working with superheater tubes made of TP347H, also known as 07Cr18Ni11Nb. It’s a high-carbon Cr-Ni austenitic stainless steel, the kind that packs a punch in terms of high-temperature strength, high-temperature plasticity, and impressive resistance to oxidation and corrosion. This stuff is the go-to choice for superheater tubes in those massive boilers, reheater tubes, steam pipelines, and petrochemical heat exchangers. It’s the heavy lifter of the materials world.
We’ve got two samples in the hot seat: the high-temperature superheater (No. 1 sample) and the medium-temperature superheater (No. 2 sample). If you take a gander at them, the high-temperature superheater looks all crimson on the outside, with a bit of loose and uneven corrosion action going on. Meanwhile, the medium-temperature superheater rocks a lighter shade, but some parts have seen the corrosive material peeling off.
Now, let’s talk environment. The high-temperature superheater has been toasting away at temperatures between 530 and 540 ℃, while the medium-temperature superheater keeps its cool at 475-480 ℃. And they’ve both been at it for a good 28,650 hours. That’s some serious time on the job.
We snipped out samples by carefully wire cutting to get the right-sized pieces for testing. The samples we’re working with measure 10 mm × 10 mm.
1.2 Testing Method
We mean business when it comes to figuring out what’s going on with these superheater pipes. Here’s how we’re doing it:
- First, we grab samples from both the high-temperature superheater and medium-temperature superheater. Then, we get these samples all prepped and ready by embedding them, sanding them down, and giving them a good polish.
- To peek at the microstructure, we bring in the ZEISS Axio Observer A3 metallurgical microscope and observe the cross-section of our samples.
- We’re curious about what these pipes are made of, so we use an EDM spectrometer to determine the material composition of both the high-temperature superheater and medium-temperature superheater.
- Next up, we’re all about hardness. We’re using a Qness Q10A+ Vickers hardness tester to figure out the microhardness of the cross-sections in our samples. We load it up with 9.8 N and give it a 10-second hold.
- The Tscan VEGA TS scanning electron microscope and an energy spectrometer are also part of the team. These instruments help us determine the microhardness and take a closer look at the cross-section morphology and composition of the samples. We’re also checking out the composition of the corrosion products on the outside of the superheater.
- For a good old strength test, we use a universal testing machine. This bad boy helps us examine the tensile properties of the samples after they’ve been through the corrosion wringer. It’s like putting these materials to the ultimate test.
So, we’re leaving no stone unturned to understand how these superheater pipes hold up after all that time in the field. It’s all in the name of science and better materials for the future.
2 Results and Discussion
2.1 Analysis of Superheater Material Composition
Now, let’s dive into the results and get to the nitty-gritty of our superheater material composition analysis.
During the long-haul shifts of the high-temperature superheater and medium-temperature superheater, they’ve had to deal with elements like Cl and S in the combustion atmosphere that can stir up high-temperature corrosion. To figure out if the composition of these tubes changed during their marathon service, we put them under the EDM spectroscope’s watchful eye.
The results are in Table 1. We’ve got No. 1, which is our high-temperature superheater, and No. 2, which is the medium-temperature superheater. And guess what? They’re both sticking to the script. Compared to the standard composition of TP347H, these tubes are right on the money. The composition checks out, which means that all that high-temperature corrosion action they’ve seen over time hasn’t messed with the material composition.
Table 1: Chemical composition of high and medium-temperature superheater tubes (mass fraction)
| Sample | C | Si | Mn | P | S | Cr | Ni | Nb |
|---|---|---|---|---|---|---|---|---|
| NO. 1 | 0.057 | 0.400 | 1.330 | 0.025 | <0.010 | 17.550 | 9.740 | 0.680 |
| No. 2 | 0.058 | 0.440 | 1.280 | 0.022 | <0.010 | 17.700 | 9.810 | 0.680 |
But here’s where it gets interesting. The fly ash from the biomass boiler’s combustion contains a hefty dose of Cl elements and alkaline metal elements. As this fly ash takes a journey through the superheater, it sticks around, clinging to the tube wall’s surface. It’s like a party for ash, where chloride, sulfide, salts, and even an oxidation film join the mix. This leads to a complex dance of physicochemical corrosion reactions, which ultimately results in high-temperature corrosion on the metal tube wall’s surface, forming an adhesion layer.
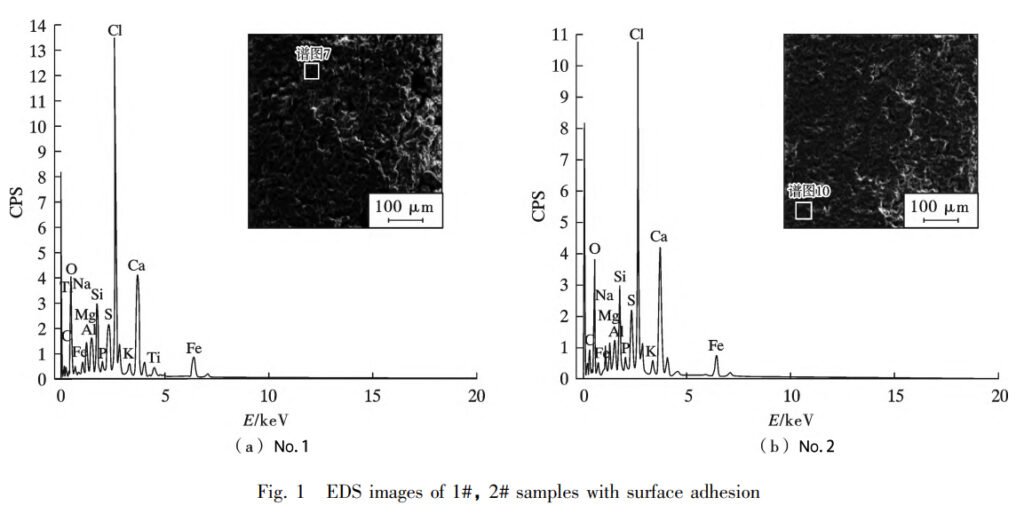
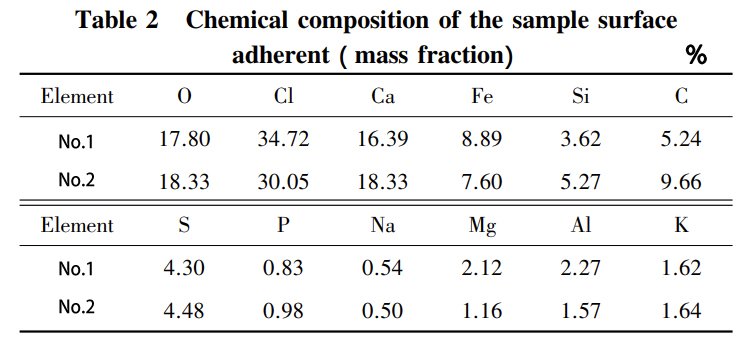
With the continuous build-up of fly ash on the tube wall’s surface, an adhesion layer forms. But this isn’t all fun and games. It’s a headache for biomass boilers because it leads to ash slagging. This not only messes with heat transfer but also provides a cozy home for corrosive elements on the surface. Moreover, it cranks up the temperature of the tube wall, which only accelerates corrosion. It’s a whole chain reaction.
Ash slagging is like the process where alkali metals and other volatile substances in the biomass go airborne under high-temperature conditions. As fly ash flows through the heated surface, it gets involved in a complex dance of gas-solid phase physicochemical processes. It condenses, sticks, or deposits on the heated surface. At the same time, some of the dust particles, in a melted or semi-molten state, end up calling the heated surface their new home. Over time, this continuous deposition of fly ash on the heated surface results in ash slagging. And guess who plays a starring role in this show? It’s the Cl element.
You see, Cl first steps up by helping alkali metal elements move from the inside of fuel particles to the surface. There, they have a good old physicochemical party with other substances. Then, Cl lends a hand by helping alkali metals vaporize and makes many inorganic compounds more mobile. So, looking at the composition of those adhesions, it’s clear that, under actual operating conditions, corrosive elements like Cl have been doing their thing on the high-temperature superheaters and medium-temperature superheaters.
In a nutshell, it’s a bit of a chemical battlefield happening in there. The composition is holding up, but the superheaters are definitely taking a hit from all the Cl and ash in the mix.
2.3 Microstructure of Corroded Pipe Wall
Now, we’re delving into the microstructure of the tube walls post-corrosion in the high-temperature superheater and medium-temperature superheater. Let’s take a peek at what’s been going on.
As you can see in Fig. 2, the outer wall side of the high-temperature superheater and the medium-temperature superheater have been taking quite a beating. The surface layer of the high-temperature superheater seems to have suffered more damage, whereas the inner wall side of both looks relatively unscathed. Here’s the scoop: The outer wall of the superheater tube gets exposed to the elements in the flue gas, and before it goes into action, a protective layer forms naturally on the metal surface. This protective layer is composed of Fe3O4 and Cr2O3, creating a dense film that’s highly resistant to corrosion. However, as the boiler operates, the flue gas packs some corrosive elements from the fly ash, and they decide to throw a party on the oxide layer’s surface. They infiltrate, create a bit of gas-phase corrosion, engage in molten salt corrosion, and even team up for localized electrochemical corrosion. This all results in the protective Fe3O4 and Cr2O3 film bidding farewell to the metal surface. And guess what? The higher the temperature, the faster this corrosion process happens. So, the high-temperature superheater’s surface has taken a more significant hit, and the tube wall’s thinning rate has accelerated.
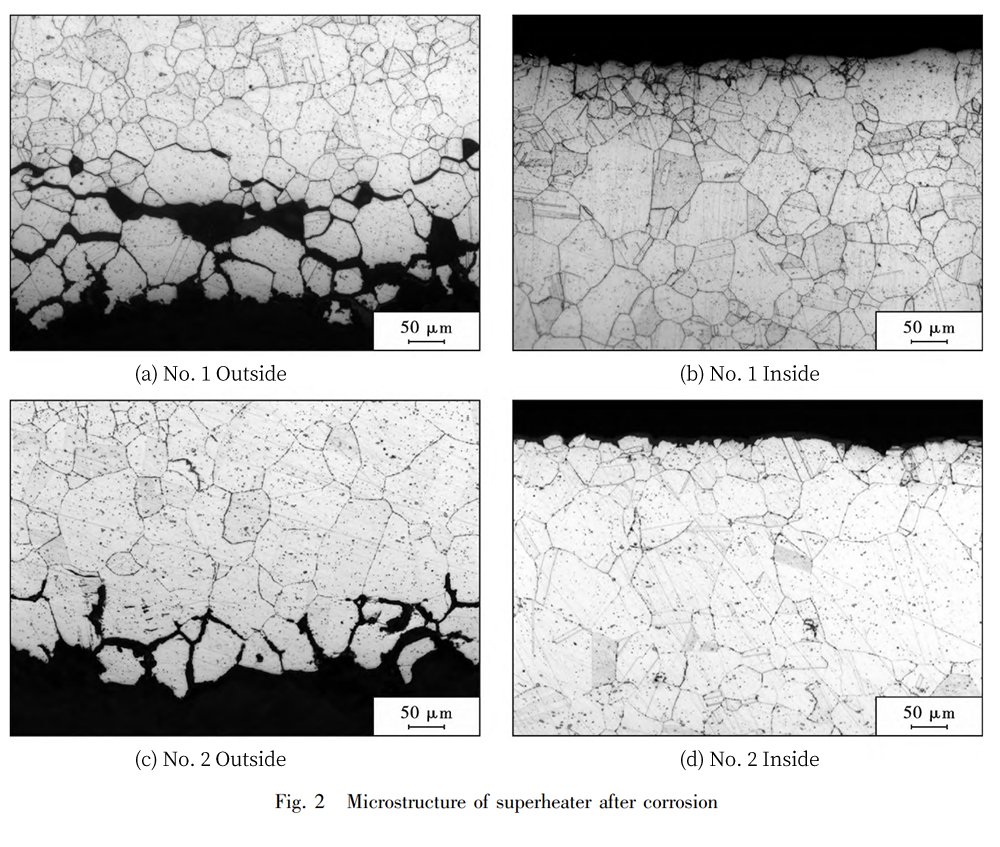
To prove that the shedding part of the metal surface layer is, in fact, the oxide layer of the protective film, we’ve done some composition testing. Take a look at Figure 3 and Table 3. You’ll see that the superheater’s surface layer mainly consists of O, Fe, and Cr elements. This confirms that the corrosive elements from the fly ash infiltrated the surface layer and went through a complex physicochemical corrosion process, which eventually caused the shedding of the oxide film from the metal surface.
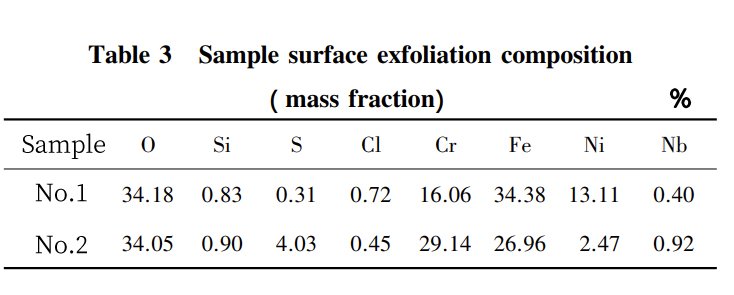
So, after a long journey of operation, the oxide layer sheds layer by layer, leading to the thinning of the superheater tube wall. And under pressure, well, you’ve got a pipe burst accident on your hands. It’s not a great situation and definitely disrupts normal production and operation.
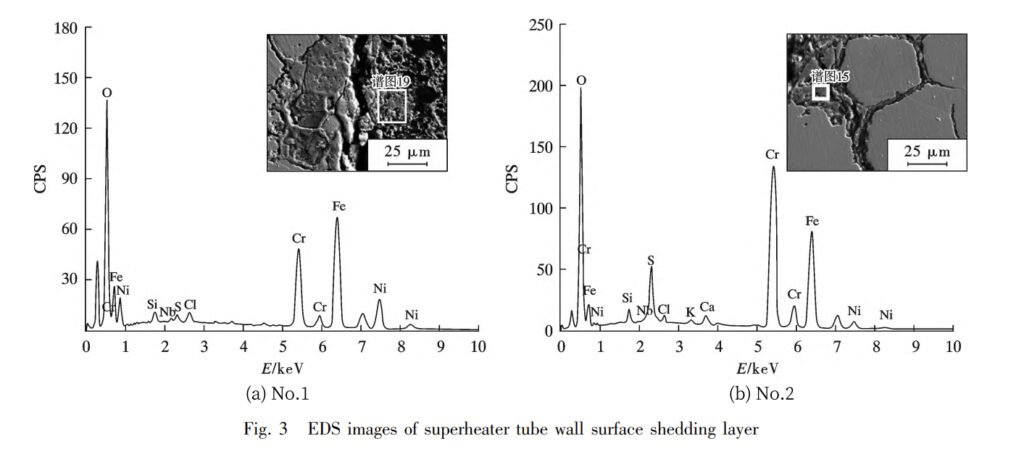
2.4 Mechanical Properties of Pipe Wall after Corrosion
Let’s dive into the mechanical properties of these pipes after they’ve endured the corrosion battle.
2.4.1 Microscopic Hardness of Tube Wall after Corrosion
In the real-world operational conditions, fly ash particles in the flue gas go to town on superheaters, thinning out those tube walls. So, for these tube walls to hold their own during long-term operation, abrasion resistance is crucial. Hardness is a key performance indicator for measuring how tough or soft a metal material is. Generally, the harder the material, the better its wear resistance. That’s why hardness values are such an important indicator for measuring a material’s ability to withstand wear.
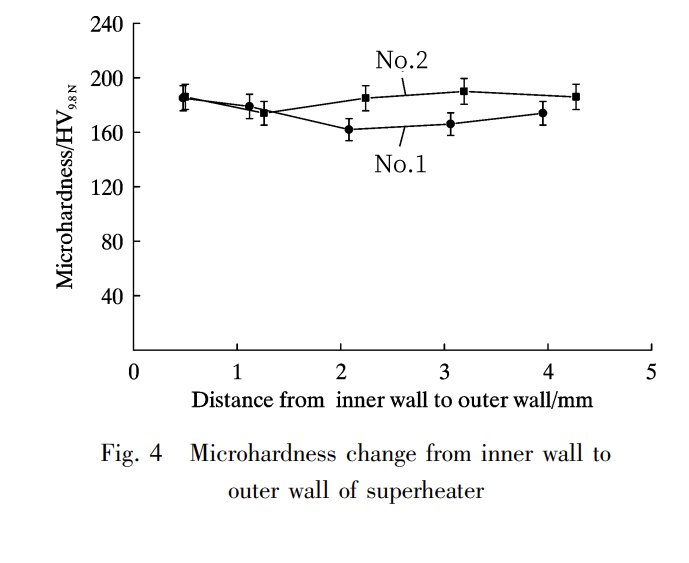
Take a look at Figure 4. It shows the micro-hardness change from the inner wall to the outer wall of the pipe walls post-corrosion in the high-temperature superheater (No. 1) and medium-temperature superheater (No. 2). What’s interesting is that the micro-hardness from the inner wall to the outer wall of both the high-temperature and medium-temperature superheaters hasn’t really changed. The average values come in at (173.20±8.66) HV9.8 N and (184.2±9.30) HV9.8 N. Even after nearly 30,000 hours of operation, the hardness of these pipe walls has stayed quite stable.
Now, the real deal is that after nearly 30,000 hours of operation, the micro-hardness of the corroded superheater pipe walls still meets the performance standards. These standards are in line with the hardness requirements set for TP347H, which is the material used for superheater pipes as per GB 5310-2008. This is quite a relief because it means that these materials are holding up pretty well.
However, there’s an interesting twist. The high-temperature superheater, after a long service life, has slightly lower hardness than the medium-temperature superheater. Why? Well, during the corrosion process, things like chloride in the sediment play a role in solid-phase corrosion of the metal’s carbide, leading to a reduction in material hardness. And guess what? High-temperature superheaters take a more significant hit from corrosion compared to their medium-temperature counterparts. So, the hardness of the high-temperature superheater’s tube wall is indeed lower, which implies that high-temperature corrosion affects the material’s performance. This, in turn, decreases wear resistance and speeds up wear and tear on the superheater tube wall.
2.4.2 Tensile Properties of Corroded Pipe Walls
The tensile test is our trusty method for figuring out the strength and plasticity of the material. It’s a fundamental approach for assessing material mechanical properties. Tensile strength, represented by Rm and Rp0.2, gives us a peek into the material’s strength. Plasticity, on the other hand, is all about elongation, which we measure with A.
Now, let’s get to the results after testing the high-temperature superheater (No. 1) and medium-temperature superheater (No. 2). Post-high-temperature corrosion, the tube wall’s tensile strength Rm is (589.50 ± 15.66) MPa for the high-temperature superheater and (642.25 ± 24.69) MPa for the medium-temperature superheater. The yield strength Rp0.2 comes in at (279.00 ± 13.02) MPa for the high-temperature superheater and (353.50 ± 30.01) MPa for the medium-temperature superheater. As for elongation A, the high-temperature superheater records (41.25 ± 3.72) %, and the medium-temperature superheater shows (45.63 ± 6.40) %.
It’s pretty clear from these results that the tensile strength, yield strength, and elongation of the high-temperature superheater, post-corrosion, are slightly lower than those of the medium-temperature superheater. This suggests that the high-temperature superheater’s wall performance is a tad less impressive after corrosion. It also drives home the point that higher temperatures lead to less impressive tube wall performance post-corrosion.
However, here’s the silver lining. Even after corrosion, the tensile properties of the superheater tubes are still up to par. They meet the tensile properties defined for TP347H in GB5310-2008 (tensile strength, yield strength, elongation of not less than 505 MPa, 205 MPa, and 35%, respectively) for superheater use. So, it’s not all doom and gloom. The overall performance of the superheater tubes is still in the game. After some protective treatment, they can keep doing their job.
2.5 Protective Measures
Alright, now that we’ve figured out what’s going on, let’s discuss some protective measures to deal with this high-temperature corrosion issue.
(1) Reduce Steam Temperature: One way to combat superheater high-temperature corrosion is to lower the steam temperature. High-temperature corrosion happens due to the corrosive elements in the flue gas, like Cl and S, engaging in complex physicochemical corrosion reactions at high temperatures. When the wall temperature is under 300 °C, the corrosive properties of the tube wall decrease significantly. However, there’s a catch here – steam temperature is directly linked to power generation efficiency. Lowering the temperature reduces power generation efficiency, which means higher production and operation costs. So, the focus often shifts to protecting the outer layer of the superheater.
(2) Regular Replacement of Superheater Pipes: Superheater pipe wall corrosion leads to thinning of the tube wall, which can result in pipe burst accidents in pressurized environments. Regularly replacing pipes can solve this problem, but it also extends the maintenance cycle and significantly raises costs.
(3) Use Corrosion-Resistant High-Temperature Alloys: While these materials are effective in resisting corrosion, they tend to be quite expensive. Choosing to use them involves weighing the cost of materials against the benefits in service life and overall effectiveness.
(4) Thermal Spraying Corrosion-Resistant Metal Coating: The thermal spraying process for creating protective coatings has seen significant development over the past decade. These protective coatings offer excellent performance, high efficiency in application, flexible and convenient equipment, and on-site construction advantages. The cost of thermal spraying is far lower than pipe replacement or using corrosion-resistant high-temperature alloys. At present, thermal spraying of corrosion-resistant metal coatings is the most widely used and economical method for addressing high-temperature corrosion of superheater tube walls.
(5) Surfacing and Laser Cladding Nickel-Based Alloys: In high-temperature, chlorinated corrosion environments such as waste incineration, traditional thermal spraying coatings may not be ideal due to their low bonding strength and high porosity, which can lead to peeling during service. To combat this issue, cladding or laser cladding technology is being used to create Inconel 625 nickel-based alloy layers, which effectively inhibit or slow down high-temperature chlorinated corrosion on water-cooled wall pipes. In the domestic market, the primary cladding technologies used are cold metal transition CMT cladding and laser cladding. However, these processes suffer from low processing efficiency, limiting their use in waste incinerator heat surfaces.
So, these are the measures we can consider to protect superheater tubes from high-temperature corrosion and ensure smooth and efficient operation.
3 Conclusion
High-temperature corrosion in superheaters poses a significant challenge for the advancement of biomass boilers. Hence, understanding the mechanisms behind high-temperature corrosion in superheaters and analyzing the changes in the performance of superheater tube walls after extended service have substantial implications. This insight is crucial for addressing corrosion issues and selecting suitable protection methods to guide the resolution of tube wall corrosion problems. Here are the key conclusions:
(1) Complex Corrosion Mechanisms: The flue gas in biomass boilers contains corrosive elements such as Cl and alkali metals, which, at high temperatures, interact with heating surfaces in various forms, including gas phase, solid phase, and liquid phase. This triggers a series of intricate physicochemical reactions, ultimately leading to the corrosion of superheater tube walls. Fly ash with corrosive elements deposits on the oxide layer’s surface, and the oxide layer experiences infiltration adhesion, thus undergoing corrosion reactions. As a result, the protective film of Fe3O4 and Cr2O3 on the tube wall’s surface peels off, causing the tube wall to thin. Importantly, the extent of corrosion and oxide layer deterioration is more severe in high-temperature superheaters due to their elevated service temperatures.
(2) Impact on Material Properties: In similar operating conditions, the micro-hardness and tensile properties of the corroded pipe wall in high-temperature superheaters are lower compared to medium-temperature superheaters. This observation underscores the fact that the high-temperature corrosive environment negatively affects the performance of the corroded pipe wall over time.
(3) Maintenance Considerations: High-temperature superheaters and medium-temperature superheaters, after prolonged service and corrosion, exhibit thinning of the tube wall and a reduction in performance. However, the composition and mechanical properties of the base material still adhere to material standards, making them suitable for continued service conditions. Protective surface treatments can be applied to prevent further corrosion-induced thinning of the tube wall, eliminating the need for measures like tube replacement. This approach extends the maintenance cycle and minimizes maintenance costs.
In summary, understanding the complex mechanisms of high-temperature corrosion in superheaters and addressing performance changes due to extended service is crucial for the effective operation of biomass boilers. By implementing protective measures and treatments, such as surface coatings, the long-term viability of superheater tubes can be ensured, reducing maintenance expenses and enhancing the overall efficiency and durability of biomass boilers.
[Source] Zhang Ping-heng, Gong Jun, Jin Jianrong, Sun Jian, Zhang Hong, Li Dai, Liu Chengwei, Lu Haifeng, Chen Guoxing, Study on High Temperature Corrosion Performance of Biomass Boiler Superheater and Protective Measures
DHB Boiler
Discover The Superior Quality And Cutting-Edge Technology Of DHB Boilers. Explore Our Range Of Biomass Boilers, Waste Heat Boilers, And More. Take Your Industrial Operations To New Heights With DHB Boiler.








